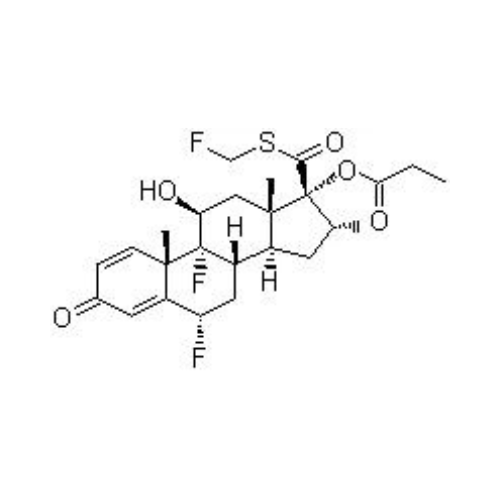
Fluticasone Propionate
Original price was: ₹150.00.₹100.00Current price is: ₹100.00.
Documentation / approvals : tdp, usdmf, edmf, cep/cos, dmf open part, dmf closed part, jdmf
It is used to treat asthma and allergic rhinitis. It exhibits excellent anti- inflammatory feature.
Application :
- Fluticasone propionate belongs to a class of drugs known as corticosteroids, specifically glucocorticoids, which are hormones that predominantly affect the metabolism of carbohydrates and, to a lesser extent, fat and protein.
- It is used to treat asthma, allergic rhinitis, nasal polyps, various skin disorders and Crohn”s disease and ulcerative colitis. It is also used to treat eosinophilic.
- Nasal spray preparation of fluticasone propionate is used in the prophylaxis and treatment of allergic rhinitis.
- Fluticasone propionate is a highly selective agonist at the glucocorticoid receptor.
- It has been shown to have a wide range of inhibitory effects on multiple cell types and mediators involved in inflammation.
Features:
- Effective
- Affordable
- Safe to use
Chemical Details:
- Formula: C25H31F3O5S
- Mol. mass: 500.57 g/mol
Product Type API
Description
| Dose | ANY |
| Product Type | API |
| Packaging Type | EXPOERT WORTHY |
| Usage | Commercial |
| Packaging Size | EXPOERT WORTHY |
Our Export Location as a Fluticasone Propionate Manufacturer, Supplier, Exporter, Stockist from India.
|
|
|
|
|
|


Reviews
There are no reviews yet.