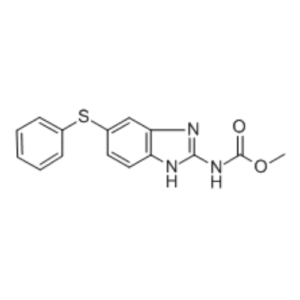
Febantel API
Original price was: ₹8,700.00.₹8,600.00Current price is: ₹8,600.00.
With the support of our adroit team of experts, we are able to offer our clients superior quality FEBANTEL. Further, it is used as a Anthelmintic agent. FEBANTEL is use in Veterinary Pharmaceutical Formulation. FEBANTEL is mainly use in combination with Praziquantel and pyrantel pamoate for Dogs. In addition, our offered FEBANTEL is processed in hygienic environment by making use of accurate amount of chemical compounds in compliance with the set industry standards. FEBANTEL is available in micronize as well as Non Micronized forms. FEBANTEL is available in IP, BP and EP.
- Pay Mode Terms: L/C (Letter of Credit),T/T (Bank Transfer),D/P,D/A
- Port of Dispatch: NAHVA SHEVA
- Production Capacity: as pr requirement
- Delivery Time: as per demand
Description
| Physical State | solid, Powder |
| Grade Standard | Medicine Grade, Industrial Grade |
| Purity (in %) | 99 |
| Packaging Size | EXPORT |
| Packaging Type | EXPORT |
Our Export Location as a Febantel API Manufacturer, Supplier, Exporter, Stockist from India.
|
|
|
|
|
|


Reviews
There are no reviews yet.