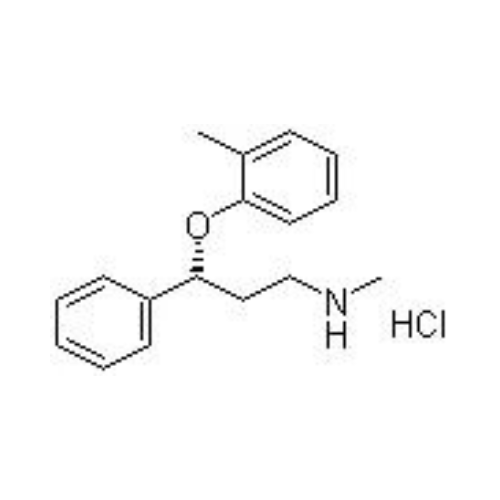
Atomoxetine Hydrochloride Api
Applications
Also known as the marketed product Strattera, atomoxetine is used with other treatment modalities (psychological, educational, cognitive behaviour therapy, etc) to improve developmentally inappropriate symptoms associated with ADHD including distractibility, short attention span, hyperactivity, emotional lability, and …
Description
Atomoxetine hydrochloride API is a white to off-white crystalline powder, a selective norepinephrine reuptake inhibitor, primarily prescribed for attention deficit hyperactivity disorder (ADHD) treatment.
Preparation
In a further aspect, the invention provides atomoxetine hydrochloride, prepared by a process comprising: a) hydrolyzing (±)-atomoxetine oxalate with a base to form atomoxetine; b) reacting the atomoxetine with an enantiomerically pure organic acid to form a salt; c) hydrolyzing the salt with a base to form …
Chemical Properties
Atomoxetine hydrochloride API is a crystalline powder with high solubility in water, known for its role as a selective norepinephrine reuptake inhibitor used in the treatment of ADHD.
Definition
Atomoxetine hydrochloride belongs to the group of alpha-sympathomimetics. The drug inhibits the reuptake of norepinephrine and dopamine. It is used for the treatment of attention deficit hyperactivity disorder (ADHD).
Description
| Product Name | Atomoxetine Hydrochloride Api |
| Country of Origin | Made in India |
| Usage | Pharma |
| Protein binding | 98% |
| Molecular Weight | 291.8 g/mol |
| Formula | C17H22ClNO |
| Melting point | 167-169°C |
| Flash point | 9℃ |
| storage temp. | 2-8°C |
| solubility | soluble in Methanol |
| form | solid |
| pka | 10.13(at 25℃) |
| color | White to Almost white |
| Water Solubility | Soluble to 50 mM in water with gentle warming |
| Water Solubility | Soluble to 50 mM in water with gentle warming |
| Packaging Size | As Per Requirement |
Our Export Location as a Atomoxetine Hydrochloride Api Manufacturer, Supplier, Exporter, Stockist from India.
|
|
|
|
|
|


Reviews
There are no reviews yet.