Albendazole
Original price was: ₹3,000.00.₹2,500.00Current price is: ₹2,500.00.
Applications
Descriptions. Albendazole is used to treat neurocysticercosis, an infection of the nervous system caused by pork tapeworms. This medicine is also used to treat cystic hydatid disease of the liver, lung, and peritoneum, an infection caused by dog tapeworms.
Description
Albendazole is an FDA-approved medication for treating a variety of parasitic worm infections. Albendazole is an antihelminthic medication with numerous indications such as cystic hydatid disease of the liver, lung, and peritoneum resulting from the larval form of the dog tapeworm, Echinococcus granulosus.
Preparation
The process comprises a) thiocyanating 2-nitroaniline of formula VI with ammonium thiocyanated in presence of a halogen to obtain 2-nitro-4-thiocyanoaniline of formula V; b)propylating 2-nitro-4-thiocyanoaniline of formula V with propylbromide in presence of n-propanol and a base in absence of a phase transfer catalyst …
Chemical Properties
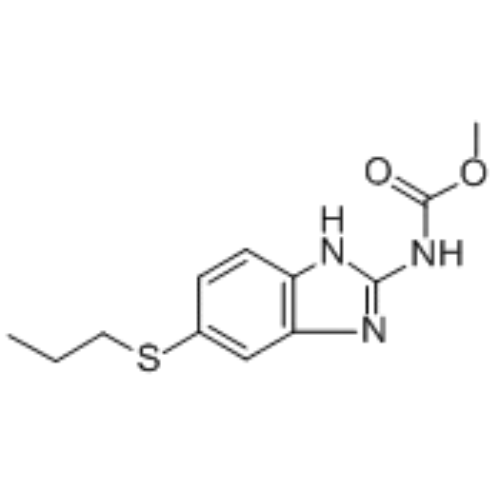
ALBENZA (albendazole) is an orally administered broad-spectrum anthelmintic. Chemically, it is methyl 5-(propylthio)-2-benzimidazolecarbamate. Its molecular formula is C12H15N3O2S. Its molecular weight is 265.34.
Definition
Albendazole is an anthelmintic drug used to treat parasitic infections caused by tapeworms and other parasites in humans and animals.
Description
| Product Name | Albendazole |
| Usage/Application | Pharma |
| Country of Origin | Made in India |
| Bioavailability | 5% |
| Melting Point | 208 to 210 DegreeC |
| CAS No | 54965-21-8 |
| Packaging Size | As Per Requirement |
Our Export Location as a Albendazole Manufacturer, Supplier, Exporter, Stockist from India.
|
|
|
|
|
|


Reviews
There are no reviews yet.