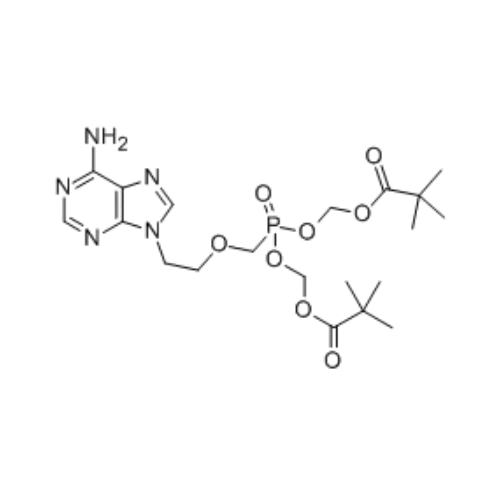
Adefovir Dipivoxil Api
Original price was: ₹2,500.00.₹2,250.00Current price is: ₹2,250.00.
Applications
Adefovir dipivoxil is a nucleoside analogue used for chronic hepatitis B infection, including against lamivudine-resistant strains. It is administered as the oral prodrug (plasma t½ 8 h, intracellular t½ of active metabolite 17 h).
Description
Adefovir dipivoxil, previously called bis-POM PMEA, with trade names Preveon and Hepsera, is an orally-administered acyclic nucleotide analog reverse transcriptase inhibitor (ntRTI) used for treatment of hepatitis B. It is ineffective against HIV-1. Adefovir dipivoxil is the diester prodrug of adefovir.
Preparation
The synthesis of adefovir dipivoxil is completed by addition of the chloromethyl pivalate to a solution of the corresponding phosphonic acid in N-methylpyrrolidone (NMP), using triethylamine as base. Figure 2. Synthetic scheme of crystalline adefovir dipivoxil.
Chemical Properties
Adefovir dipivoxil API is a white to off-white crystalline powder with low solubility in water, recognized for its antiviral properties in treating chronic hepatitis B by inhibiting viral DNA polymerase.
Definition
Adefovir dipivoxil API is an organic compound used in the treatment of chronic hepatitis B, functioning as a nucleotide analogue inhibiting viral DNA replication.
Description
| Product Name | Adefovir Dipivoxil Api |
| Country of Origin | Made in India |
| Usage | Pharma |
| CAS ID | 142340-99-6 |
| Formula | C8H12N5O4P |
| Molar Mass | 273.186 g/mol |
| Protein Binding | 4% |
| Elimination half-life | 7.5 hrs |
| Molecular Formula | C20H32N5O8P |
| Molecular Weight | 501.47 |
| Melting point | 98-102°C |
| Boiling point | 641.0±65.0 °C(Predicted) |
| Density | 1.35±0.1 g/cm3(Predicted) |
| storage temp | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | ethanol: soluble50mg/mL |
| pka | 4.16±0.10(Predicted) |
| form | solid |
| color | White to Off-White |
| Packaging Size | As Per Requirement |
Our Export Location as a Adefovir Dipivoxil Api Manufacturer, Supplier, Exporter, Stockist from India.
|
|
|
|
|
|


Reviews
There are no reviews yet.