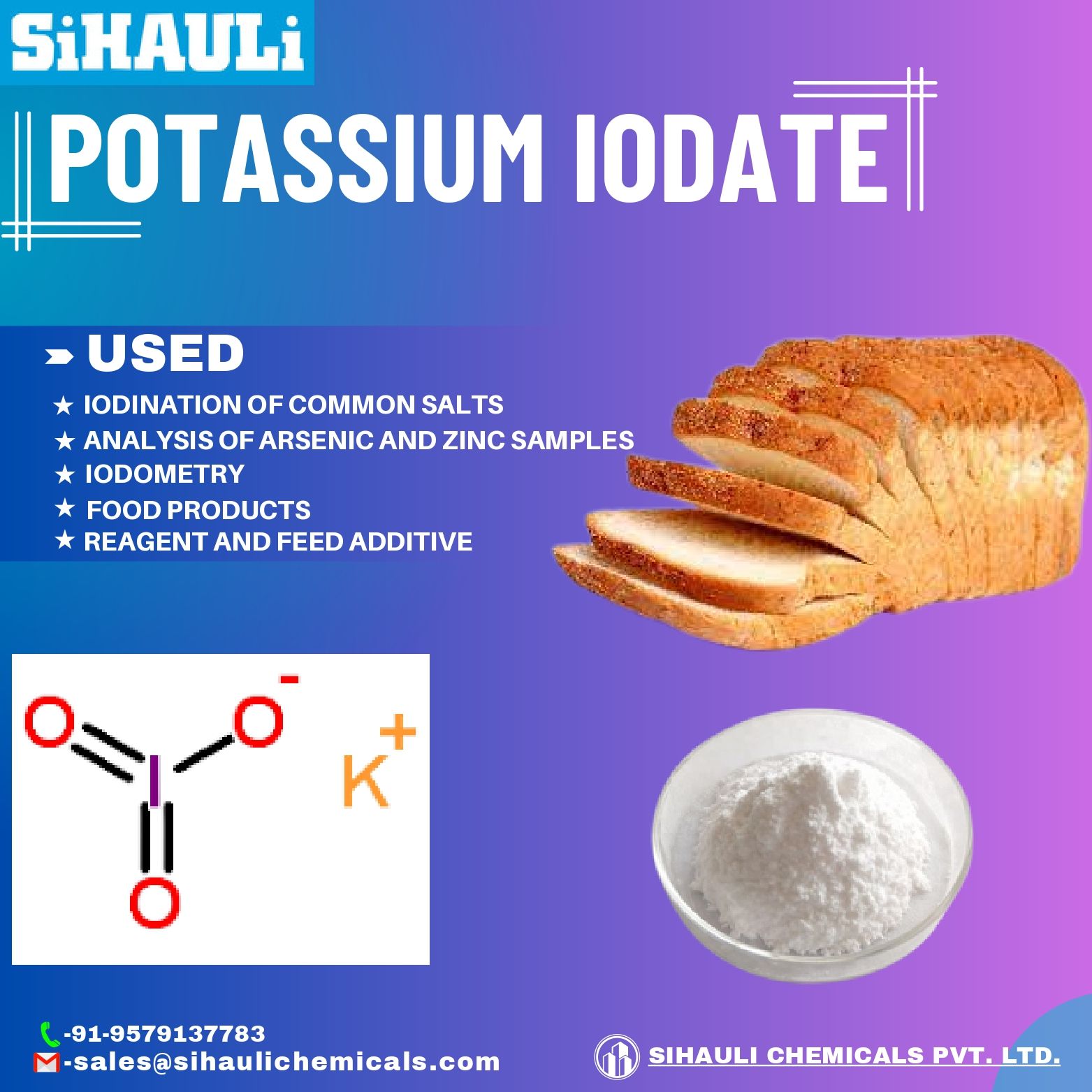
Potassium iodate is the major chemical compound used for the iodization of edible common salts. It provides a convenient way of performing iodometric work. Potassium iodate is also sometimes used to precipitate thorium especially to remove it from the rare earth elements.
Potassium Iodate (KIO3) is an ionic compound made of Potassium cation (K+) and Iodate anion (IO3–). It is one of the most commonly used compounds for the iodization of table salt. Potassium Iodate can be prepared by potassium base with iodic acid or potassium hydroxide iodin. During titration reaction, it can be used to standardize Na2S2O3 solution. In some parts of the world, it is even used in baking.
Uses of Potassium Iodate
Potassium Iodate has multiple applications in diverse fields. Some of its common uses are discussed below.
- It is used in the salt industry to add iodine to sodium chloride. Under wet conditions, iodide is oxidized to iodine, which is then added to the salt. In this way, table salt is iodized in the salt industry.
- It is used in baking. It helps to condition the dough which makes bread soft and fluffy. It is often regarded as a substitute for baking soda due to this reason.
- Potassium Iodate is also used as a maturing agent in the food industry. So, it is often used as a food additive.
- Potassium Iodate is used in the testing of arsenic and zinc.
- It is used in iodometric reactions.