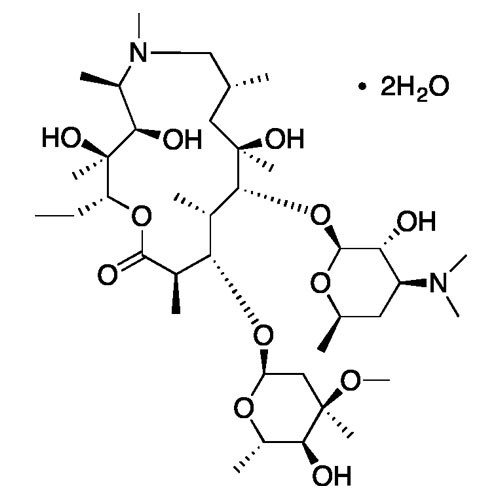
Azithromycin Dihydrate
Original price was: ₹8,000.00.₹7,000.00Current price is: ₹7,000.00.
Applications
Azithromycin is used to treat certain bacterial infections, such as bronchitis; pneumonia; sexually transmitted diseases (STD); and infections of the ears, lungs, sinuses, skin, throat, and reproductive organs
Description
It is primarily used for the treatment of respiratory, enteric and genitourinary infections and may be used instead of other macrolides for some sexually transmitted and enteric infections. It is structurally related to erythromycin 2.
Preparation
Azithromycin monohydrate (5g) was suspended in 50 ml of water/acetone (30:70 by weight) and stirred for 24 hours at 20-25°C. The solid was collected by filtration, and dried at 35-40°C to give azithromycin dihydrate (4.8g).
Chemical Properties
Azithromycin dihydrate is a white crystalline powder with low solubility in water, known for its antibiotic properties and commonly used to treat bacterial infections.
Definition
Azithromycin tablets are a macrolide antibacterial drug indicated for mild to moderate infections caused by designated, susceptible bacteria: • Acute bacterial exacerbations of chronic bronchitis in adults (1.1)
Description
| Product Name | Azithromycin Dihydrate |
| CAS Number | 117772-70-0 |
| Grade | Pharma Grade |
| Manufacturer | Sihauli Chemical Limited |
| Usage/Application | Antibiotics used for a variety of sensitive bacterial infections |
| Chemical Formula | C38H76N2O14 |
| Molecular weight | 785.02 g/mol |
| Made In | India |
| Packaging Size | As Per Requirement |
Our Export Location as a Azithromycin Dihydrate Manufacturer, Supplier, Exporter, Stockist from India.
|
|
|
|
|
|



Reviews
There are no reviews yet.